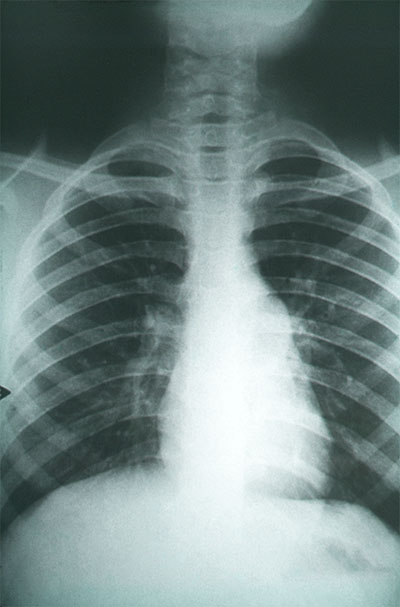
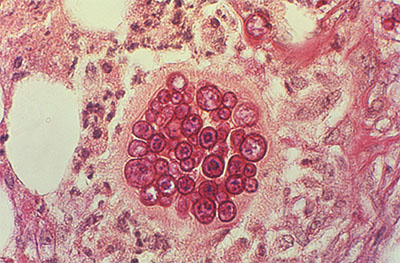
Coccidioidomycosis is a serious form of fungal pneumonia that predominantly affects people who visit or live in the southwest United States and northern Mexico. Within the endemic zone, it can be extremely common. For example, in Arizona, coccidioidomycosis is responsible for approximately 25% of all community-acquired pneumonia cases. The pathogen is present in the soil, and even immunocompetent individuals can become infected after breathing in the fungal spores. The majority of coccidioidomycosis cases will resolve favorably within weeks to months. But some cases require anti-fungal treatment – life-long treatment for some patients. And in some cases, the infection is fatal even with anti-fungal treatment.
Diagnostic challenges
Current diagnostics for coccidioidomycosis include fungal culture, microscopy, and serology (detection of patient antibodies). These tests have limitations that include cost, time to result, and limited sensitivity. They all require specialized equipment and user expertise. Consequently, most tests are performed on specimens shipped to reference laboratories and delays for diagnosis are common. The vast majority of coccidioidomycosis patients endure symptoms for over three weeks before receiving their diagnosis. During this time, they frequently receive inappropriate (and ineffective) antibiotic prescriptions and can be subjected to unnecessary medical procedures. These diagnostic challenges and delays result in poor patient care, poor antimicrobial stewardship, and increased healthcare costs.
DxDiscovery solution
Our solution is to produce a coccidioidomycosis antigen-detection immunoassay that is rapid, inexpensive, and usable at or near the point of care. The approach is detection of Coccidioides cell wall polysaccharides from minimally invasive specimens, such as urine or serum. To address different end user needs across the spectrum of health care workers who encounter coccidioidomycosis patients, we are currently developing both a kit-based ELISA, which could be easily used in most hospital laboratories, and a lateral flow immunoassay (like the at-home COVID-19 test), which could be deployed by front-line clinicians directly at the point-of-care (e.g. urgent care centers and primary care clinics). Both options would increase test accessibility and provide actionable diagnostic information without the need to ship specimens to a reference laboratory. The goal is to enable prompt diagnosis of coccidioidomycosis and the swift initiation of appropriate patient care.
Market opportunity
There are approximately 140,000 cases of coccidioidomycosis in the U.S. each year, and coccidioidomycosis should be considered for all individuals with community acquired pneumonia in endemic regions. Thus, the serviceable available market is conservatively estimated at approximately 560,000 tests per year in the United States. This estimate is likely a strong underestimate since it does not include individuals who travel to endemic regions (e.g. tourists to Arizona, New Mexico, or the central valley of California).
R&D funding
DxDiscovery has been awarded Small Business Innovation Research (SBIR) Phase I and Phase II contracts from the National Institute Allergy and Infectious Disease for development of the coccidioidomycosis immunoassay.
For more information, please contact us: ContactUs@DxDiscovery.com